usp class vi vs fda
In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600.
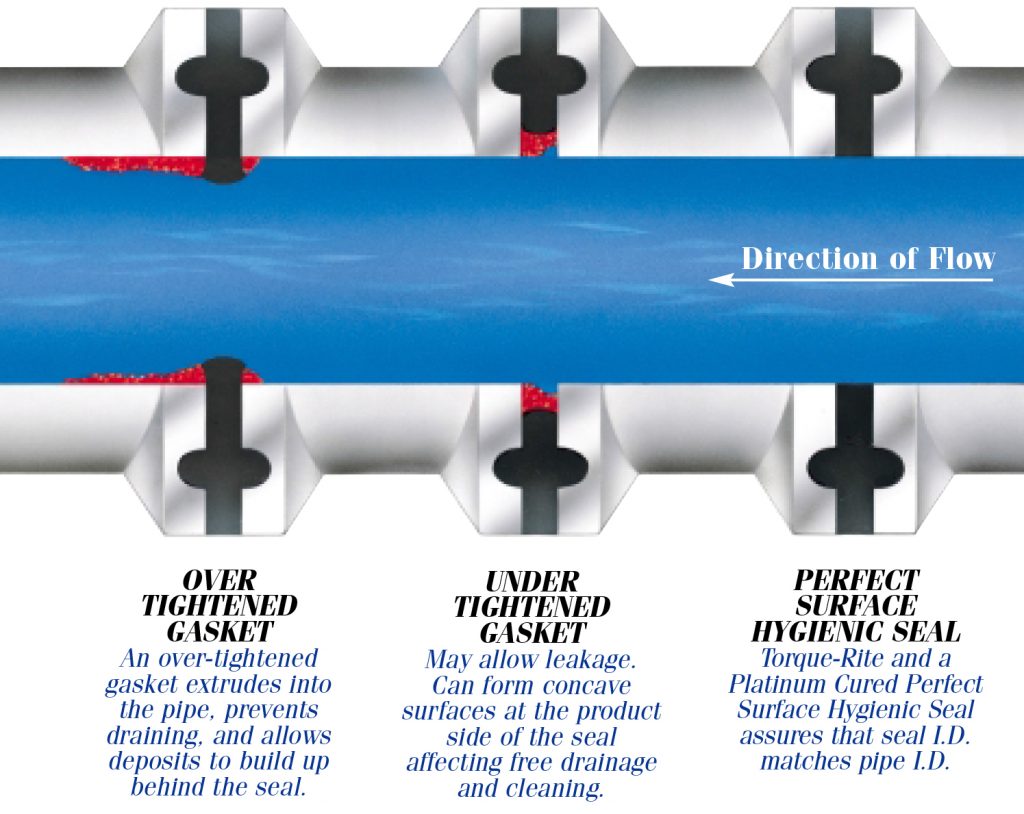
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa
Most importantly use of Class VI certified materials substantially reduces the risk of.

. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. Among all USP classes Class VI materials meet the most stringent testing requirements. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.
To begin the USA food and Drug Administration FDA places. The USP Class VI compounds must be. Class VI testing is aimed to.
In order for a compound to pass USP Class VI standards it must. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. For filters composed primarily of plastic parts the.
FDA food-grade rubber materials typically comply with FDA 21. Typical applications for our FDA NSF. 27 rows The US.
The FDA requires testing of finished devices however the demonstration of biocompatibility of materials according to USP Class VI standards is provided as an aid to device manufacturers. Sil 714001 USP class VI Silicone 1 70 Yes transl. It generally ensures a high quality level and better acceptance with the FDA and USDA.
Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. Since 2002 the standards have been published annually although prior to that it was as infrequent as every 10 years. Specially formulated for long term sealing.
USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600 Specially. Because neither USP Class VI nor ISO 10993 are synonymous with. Moulded O-rings class 1 less than 10 furnace black These can be produced in all.
Initially it is applied at 50C 122F for 72 hours then at 70C 158F for 24 hours and lastly at 121C 250F for 1 hour. However Class VI also requires subacute toxicity and. There are six classes VI being the most rigorous.
There may be some confusion between FDA USP Class VI and FDA food grade materials. USP Plastic Class VI as this group is also known includes silicones that have. For example USP Class VI requires an intracutaneous irritation test which is also required for ISO 10993 compliance.
Class VI materials which were discussed earlier are tested according to the above protocols. Sil 714002 USP class VI Silicone 1 70 Yes transl.

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket

Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket
![]()
Usp Class Vi Silicone Is Independently Certified For Biocompatibility Specialty Silicone Products Inc

What Is Usp Class Vi Testing Tbl Plastics

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Fda And Usp Class Vi O Rings Guide 2020 Nes

Usp Class Vi Foster Corporation

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Material Selection Medical Injection Molding Xcentric Mold
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Usp Class Plastics Pacific Biolabs

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants


